
BACKGROUND: Allogeneic haematopoietic stem cell transplantation (allo-HCT) is a standard treatment for relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL). However ~30-40% of patients (pts) still relapse after HCT. We report a cohort of 20 r/rB-ALL pts, who relapsed after HCT, and enrolled in the CAR2.0 study receiving one or two types of CAR-T cells targeting various B-ALL antigens.
METHOD: Pts with r/r B-ALL who relapsed after allo-HCT and did not have significant active comorbiditeis, were enrolled in the study. The target antigens were determined based on immunostaining of each pt's leukemia cells, and CAR-T infusions included a single, or a combination of CAR-Ts targeting the following antigens: CD19, CD22, CD123 and CD38. T cells were collected from pts (N=4) or their allogeneic donors (N=16) and transduced with an apoptosis-inducible, safety-engineered lentiviral CAR with the following intracellular signaling domains: CD28/CD27/CD3ζ-iCasp9 (4SCAR). Pts received cyclophosphamide/fludarabine lymphodepleting therapy before infusion of 0.2-5.8x106 CAR-T/kg per infusion. In addition to disease response, we carefully monitored the quality of apheresis cells, efficiency of gene transfer, T cell proliferation rate, CAR-T infusion dose, and the CAR-T copy number in peripheral blood.
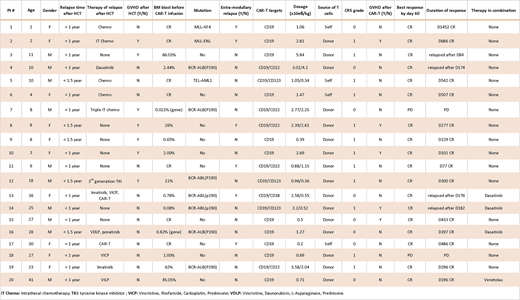
RESULTS: Among the 20 enrolled pts, 11 were <18 years of age, and 7 were BCR- ABL (P190) positive. Before CAR-T treatment, 7 pts had ≤grade 2 active graft-versus-host disease (GVHD), and 13 pts received chemotherapy or targeted therapy after their relapse post HCT. Six pts had extramedullary relapse and 2 of them also had bone marrow relapse. The tumor burden in bone marrow ranged from minimal residual disease (MRD) negative to 66% of blasts, based on flow cytometry before CAR-T therapy. Five pts had >10% blasts in bone marrow, 8 pts had <3% blasts, and 7 pts had MRD negative bone marrow (summarized in the Table below). Based on the GVHD history, chimerism state and the available T-cell sources, 16 pts used allogeneic HCT donor T-cells for CAR-T preparation. All pts were full donor chimeras prior to CAR-T infusion, except one pt who had 41% donor cells in bone marrow. Eleven pts received a single CD19 CAR-T infusion, with a mean dose of 1.6x106 CAR-T/kg, and ten achieved an MRD remission and one had progressive disease (PD) within 60 days by flow cytometry. The remaining 9 pts received 2 CAR-Ts (CD19 plus CD22, CD123 or CD38 CAR-Ts) given on the same day, and resulted in 8 CR and 1 PD within 60 days. After CAR-T infusion, no cytokine release syndrome (CRS) was observed in 8 pts, and 12 pts experienced CRS of grade 1, which was consistent with the previously described low toxicity profile of the 4SCAR design. Acute GVHD ≤ grade 2 developed in 5 pts within one month following CAR-T cell infusion but all responded well to supportive care and/or cyclosporine infusion. The 2 pts who developed PD after CAR-T infusion included the one with 41% donor chimerism and had grade 2 GVHD and active infections before CAR-T infusion. The other pt with PD following CAR-T had severe bone marrow suppression, low leukocyte count, infections and was transfusion dependent before enrollment. This emphasizes the need for controlling comorbidities before infusion of CAR-T cells. In summary, total 18 patients (90%) achieved negative MRD remission within 2 months of therapy with acceptable CRS. Four pts relapsed (after being in remission for 3 months) and 14 pts are in continued remission, 6 of which for > 1 year. None of these 20 pts received a second HCT after CAR-T infusion. GVHD developed in 5/16 (31%) pts after donor source CAR-T cell infusion within one month, but all responded well to treatment.
CONCLUSION: This study focuses on CAR-T cell therapy following relapse after HCT. While the expanded study is ongoing, we present results of the first 20 pts. Use of donor-derived or recipient-derived CAR-T products in pts who relapsed after allo-HCT is well tolerated and it may prolong life expectancy of these pts while maintaining good quality of life.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal